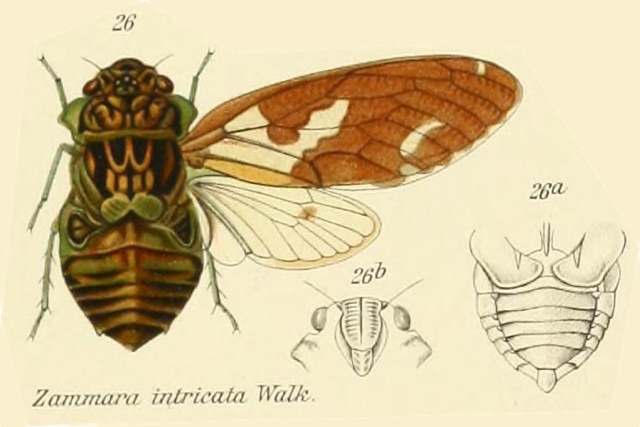
Odopoea degiacomii Distant, 1912, was described by British entomologist W. L. Distant in 1812.
Odopoea degiacomii is a visually impressive cicada with a prominent pronotal collar that should inspire thoughts of Dracula the Vampire (like other members of the cicada tribe Zammarini). It won’t suck your blood, but it will suck xylem sap from trees.
This cicada is found in the Dominican Republic and probably Haiti.
Scientific classification:
Family: Cicadidae
Sub Family: Cicadinae
Tribe: Zammarini
Genus: Odopoea
Species: Odopoea degiacomii Distant, 1912
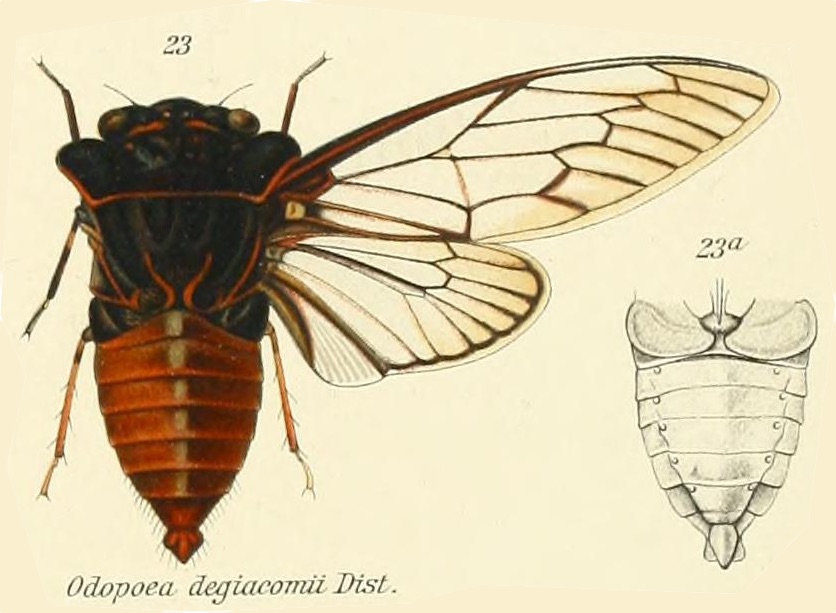
An Odopoea degiacomii Stål genus description by W. L. Distant from Genera Insectorum:
Characters. — Head (including eyes) about equal in width t.. base of mesonotum, ocelli a little wider apart from eyes than from each other, eyes prominent, a little passing the anterior pronotal angles; face more or less longitudinally sulcate; rostrum about reaching the posterior cox*; pronotum shorter than mesonotum, the lateral margins angularly ampliate; mesonotum (including basal cruciform elevation) almost as long as head and pronotum together; abdomen broad, centrally ridged, the lateral areas more or less oblique, about as long as space between apex of head and base of cruciform elevation; operçula short, broad, not extending beyond base of abdomen; tympanal coverings outwardly complete, the orifices only exposed inwardly; tegmina three or more than three times as long as broad, apical areas eight; wings with six apical areas.
References:
- The illustration comes from the journal Genera Insectorum, and a specific article from 1914 by W. L. Distant titled Homoptera. Fam. Cicadidae, Subfam, Gaeaninae. Read it on the Biodiversity Heritage Library website.
- Species name information/verification comes from Allen Sanborn’s Catalogue of the Cicadoidea (Hemiptera: Auchenorrhyncha).